The Paralisa™ Advantage
IgG1 ELISA for the testing of Johne’s disease (paratuberculosis) in deer and cattle.
Commercially available laboratory kits for diagnosis of Johne’s Disease use a single antigen absorbed ELISA, this test is validated for use with milk or serum. While this single antigen approach provides reliable detection of the most positive animals, there is more to gain from identifying the early, subclinical (infectious) cases within the herd.
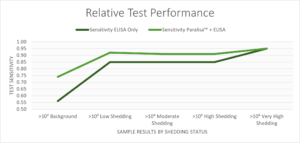
Test sensitivity data for two serological ELISA tests – IDEXX and Paralisa™, considered singly and in combination, for a range of shedding threshold cutoffs determined using qPCR.
The Paralisa™ serum test established by the University of Otago at the Disease Research Limited (DRL) laboratory is a multi-antigen ELISA test. When used in parallel with the screening kit on serum samples, Paralisa™ improves test sensitivity, allowing better management decisions to be made on farm. The greatest gains are in the early, subclinical stages of disease, where shedding animals can be identified before production losses set in. By identifying these animals early, farms can guard against further spread of the disease and recover testing costs by strategic culling.
Identifying cows with advanced disease (high shedding) and culling those cows immediately relieves some of the burden of bacterial spread but can leave behind the animals that are still in the infected and infectious states. Take advantage of the Paralisa™ test to detect more animals which have mounted an immune response, before they begin losing production and shedding bacteria throughout the herd. To see how early detection, and selective culling, helped one Canterbury dairy farm, find our published article here.
Bookings
Samples should be booked in by phone or email prior to sending. Booked-in samples will be given priority.
Tel: 03 489 4832 ¦ Email: drl@drl.net.nz ¦ Simon: 021 249 7710
Sample submission
One red or purple top tube is required for each Paralisa™ test, clearly identifying the animal the sample has come from. Please ensure that your samples are accompanied by a completed Submission Form. Please read our terms and conditions prior to sending your samples.
Reporting results
Expected turnaround time for results is 5–7 working days. If results are urgent, please let the laboratory know at the time of booking.
Results will be reported as Negative (Neg), Low Positive (L Pos), Moderate Positive (M Pos), or High Positive (H Pos).
Results will be provided to the vet involved, if indicated by the farmer. No other agency will receive the data.
